Vaccines
In 1998, the FDA licensed the LYMErixTM vaccine against Lyme disease. LYMErixTM contains OspA, the main causing agent of Lyme disease. Three doses of the vaccine are injected in people 15 to 70 years old. The second dose is injected one month after the first and the third dose is injected one year after the first. Vaccine administration should be timed so the second and third dose are injected several weeks before the start of B. burgdorferi transmission season in April.
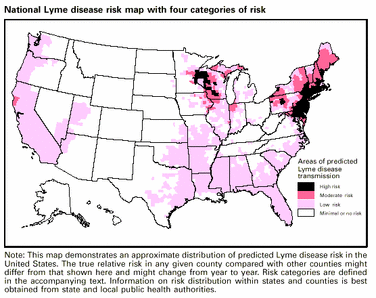
The vaccine was targeted at those at risk for exposure to infected ticks and this risk should be analyzed according to the distribution of the disease and the extent a person's activities place them in contact with ticks.
In 2002, the LYMErixTM vaccine was withdrawn from the market due to poor sales and as a result, various other preventive measures are available. In particular, there is a focus on other vaccines that require fewer doses and provide long term protection. A key interest has been in developing vaccines that specifically target the desired tick points of Lyme disease and protect against multiple pathogens with one vaccine.
The vaccine was targeted at those at risk for exposure to infected ticks and this risk should be analyzed according to the distribution of the disease and the extent a person's activities place them in contact with ticks.
In 2002, the LYMErixTM vaccine was withdrawn from the market due to poor sales and as a result, various other preventive measures are available. In particular, there is a focus on other vaccines that require fewer doses and provide long term protection. A key interest has been in developing vaccines that specifically target the desired tick points of Lyme disease and protect against multiple pathogens with one vaccine.
